Hit Biophysical Characterization
After going through a drug discovery phase such as high-throughput screening (HTS), it is crucial to obtain more information to guide the drug candidate selection and optimization for the next step. Biophysical assays bring a lot of valuable information for characterization and prioritization of hit compounds. In fact, these biophysical techniques are applied at various stages of drug discovery, for example, assisting determine binding kinetics and thermodynamics; providing important supplements to data on biochemical and cellular activity as well as solubility, aggregation, and cell permeability; and providing fragment-based screening (FBS) approaches in fragment-based drug discovery (FBDD).
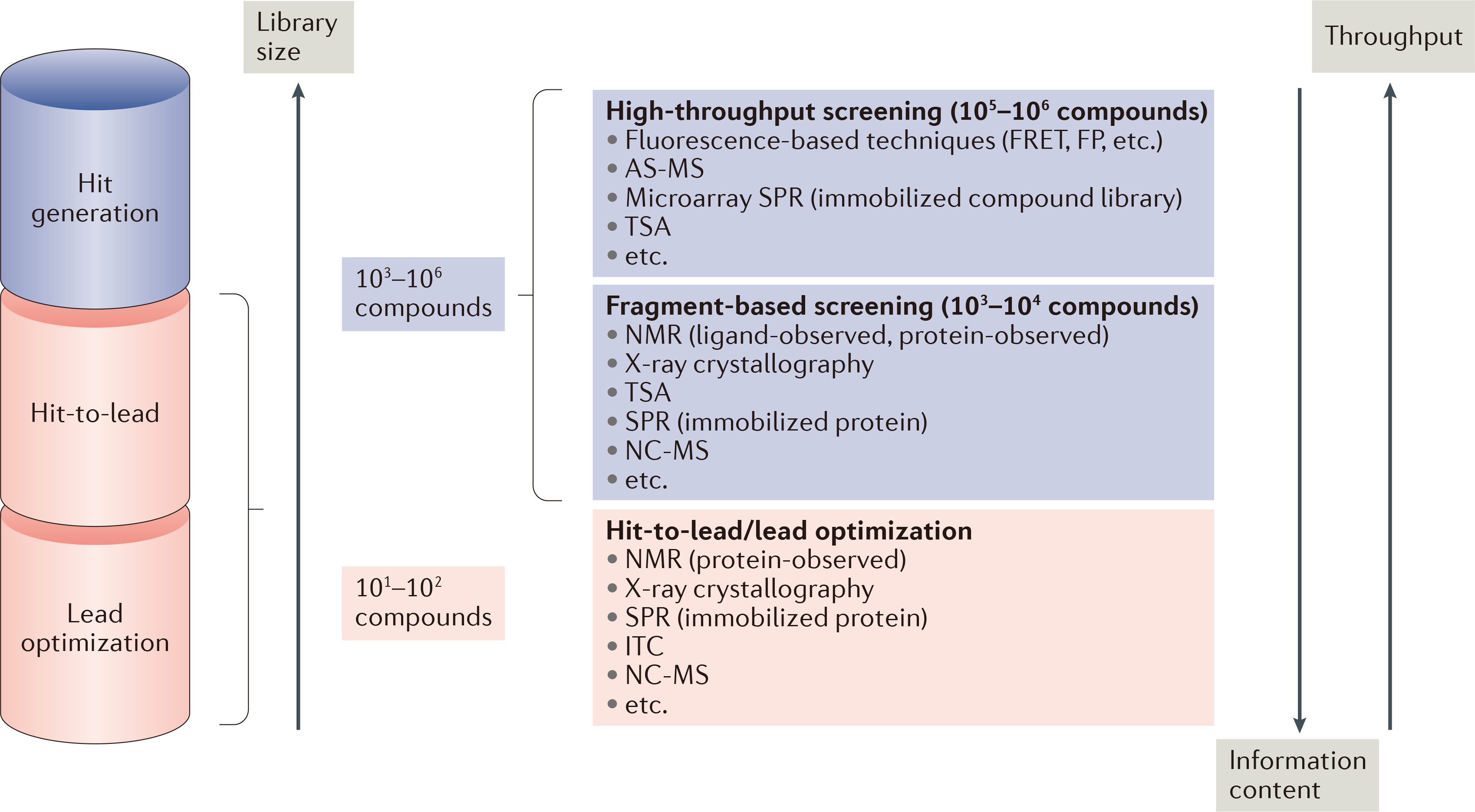 Figure 1. Biophysical techniques in drug discovery. (Renaud J P.; et al. 2016)
Figure 1. Biophysical techniques in drug discovery. (Renaud J P.; et al. 2016)
With expertise and experience in biophysical techniques, Creative Biostructure provides more rational and rigorous solutions for hit confirmation, compound characterization, and targeted-ligand binding mode analysis for customers with drug discovery requirements. We are able to determine the atomic details of target-ligand interactions and identify the ligand binding site on the target using X-ray crystallography, NMR spectrometry, or cryo-electron microscopy (cryo-EM). Isothermal titration calorimetry (ITC) and surface plasmon resonance (SPR) are utilized to provide quantitative data on the binding kinetics and thermodynamics, which imply information about the mode of action of hit compounds. These approaches offer a comprehensive data package that can increase confidence in the selection of hits for the following optimization phase. We can also provide many novel and effective biophysical approaches, and our well-trained team of experts can combine different approaches according to the specific information of the project to compensate for the limitations of a single technology.
Our Hit Biophysical Characterization approaches include, but are not limited to:
- X-ray crystallography (protein-ligand co-crystallization or crystal soaking)
- Saturation transfer differences (STD) NMR
- Thermal shift assay (TSA)
- Surface plasmon resonance (SPR) spectroscopy
- Bio-layer interferometry (BLI)
- Isothermal titration calorimetry (ITC)
- Differential scanning calorimetry (DSC)
- Quartz crystal microbalance (QCM)
- Microscale thermophoresis (MST)
- Dynamic light scattering (DLS)
- Mass spectrometry (MS)
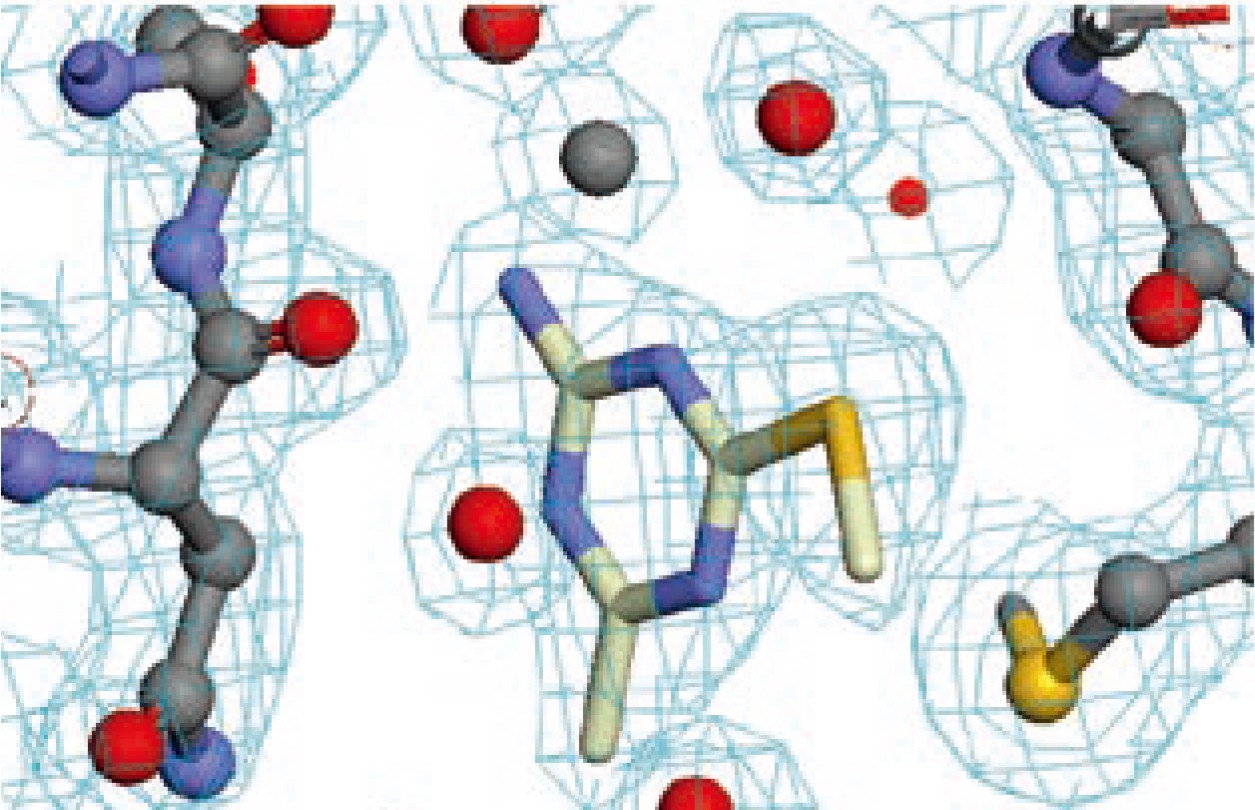 | Strengths: Identifies the binding site and visualizes protein-ligand interactions at atomic resolution for lead structural optimization. |
| Limitations: Requires high-quality crystals suitable for co‑crystallization or soaking and no kinetic information is directly obtained. |
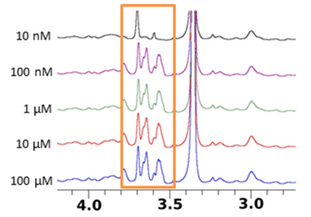 | Strengths: High sensitivity for low-affinity ligands and allows for the analysis of fragments. |
| Limitations: No information on the binding mode and cannot directly distinguish between specific and non-specific binding interactions. |
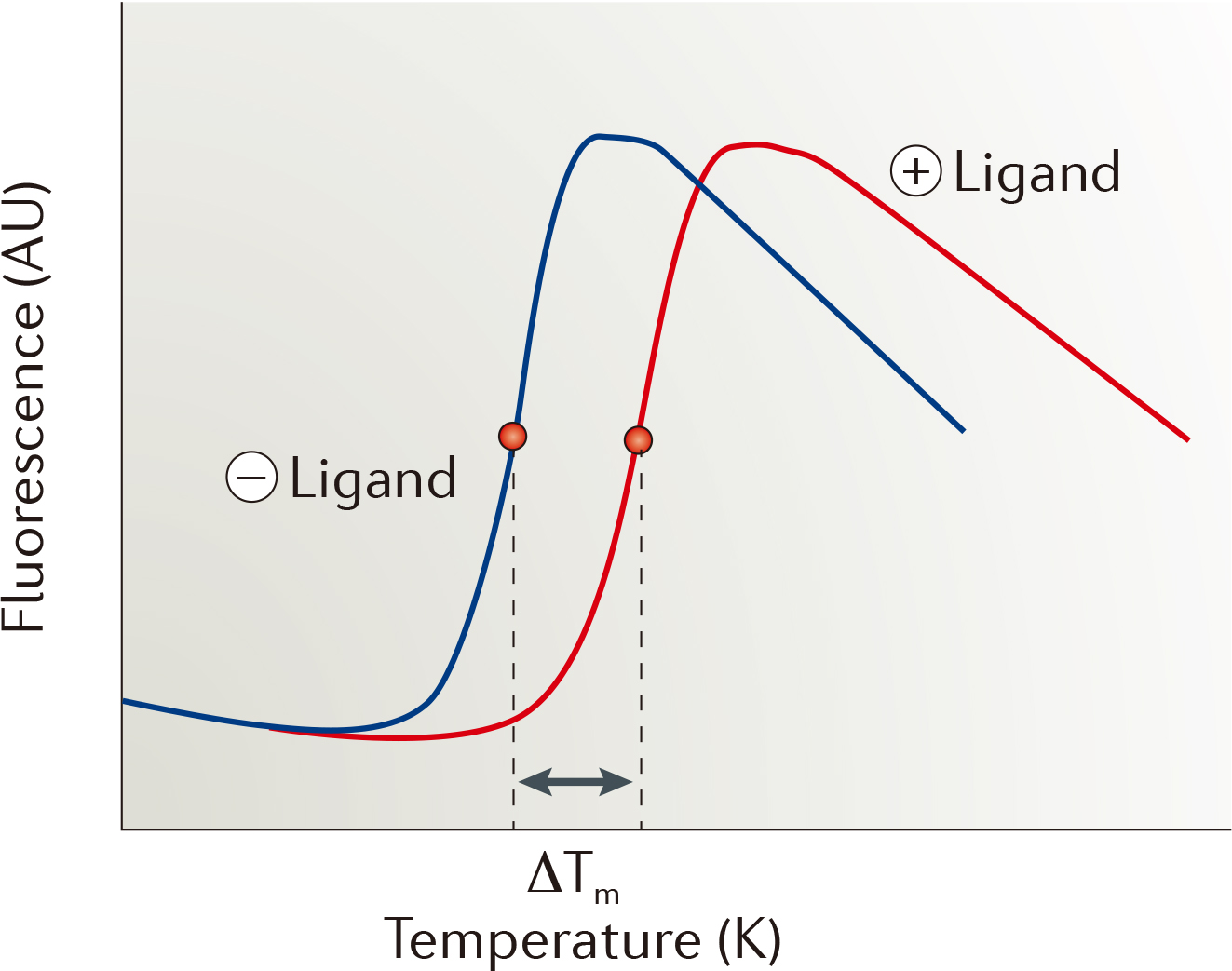 | Strengths: The assay development process is fast and robust. We provide various types of TSA approaches for monitoring protein thermal stability. |
| Limitations: Not applicable to hydrophobic or disordered proteins, and artefacts occur due to fluorescence quenching or aggregation. |
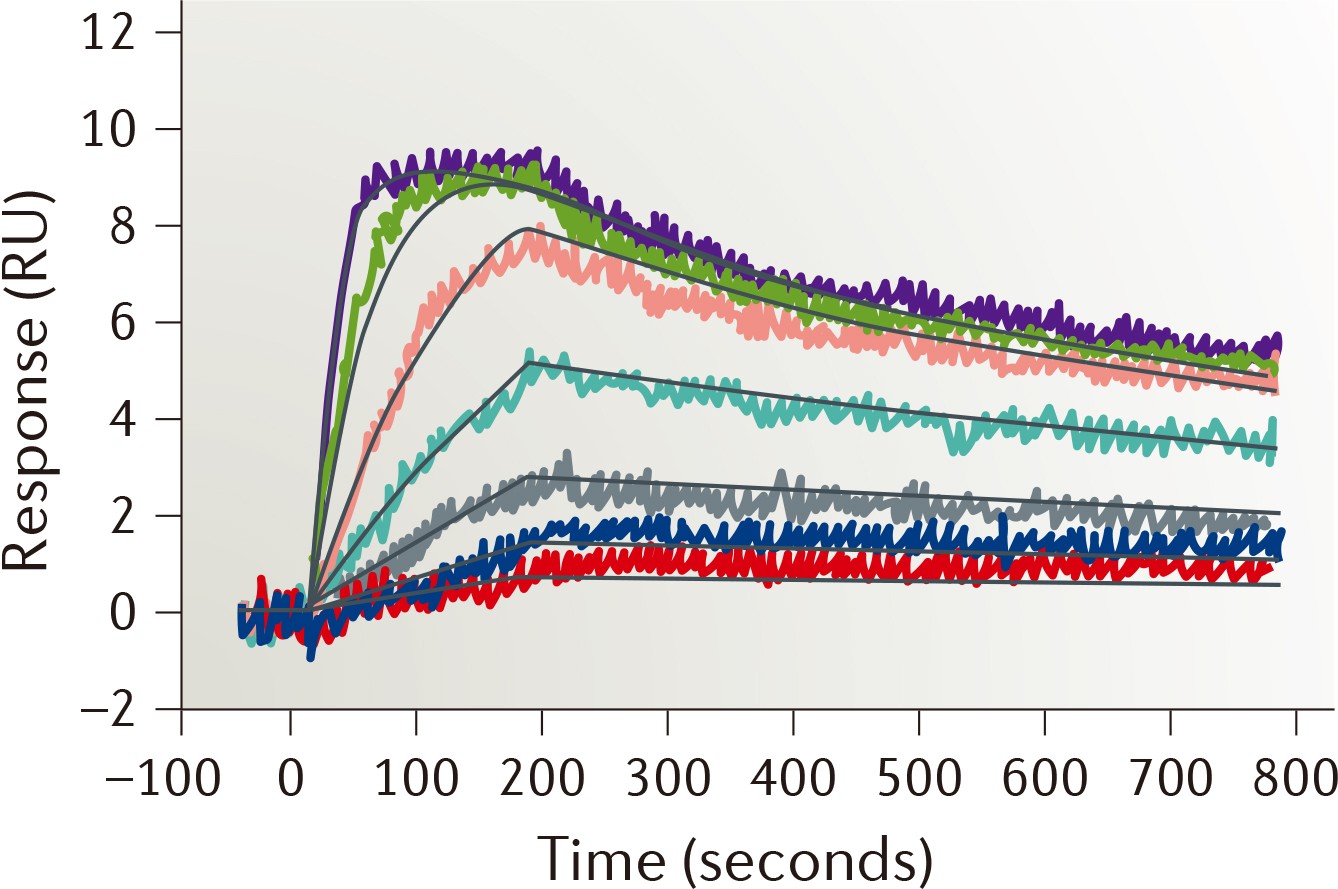 | Strengths: Time-resolved measurements of interactions can be performed directly, and the high sensitivity supports the analysis of fragments. |
| Limitations: Requires immobilization of target protein with high stability, and signals could be affected by solvent effects. |
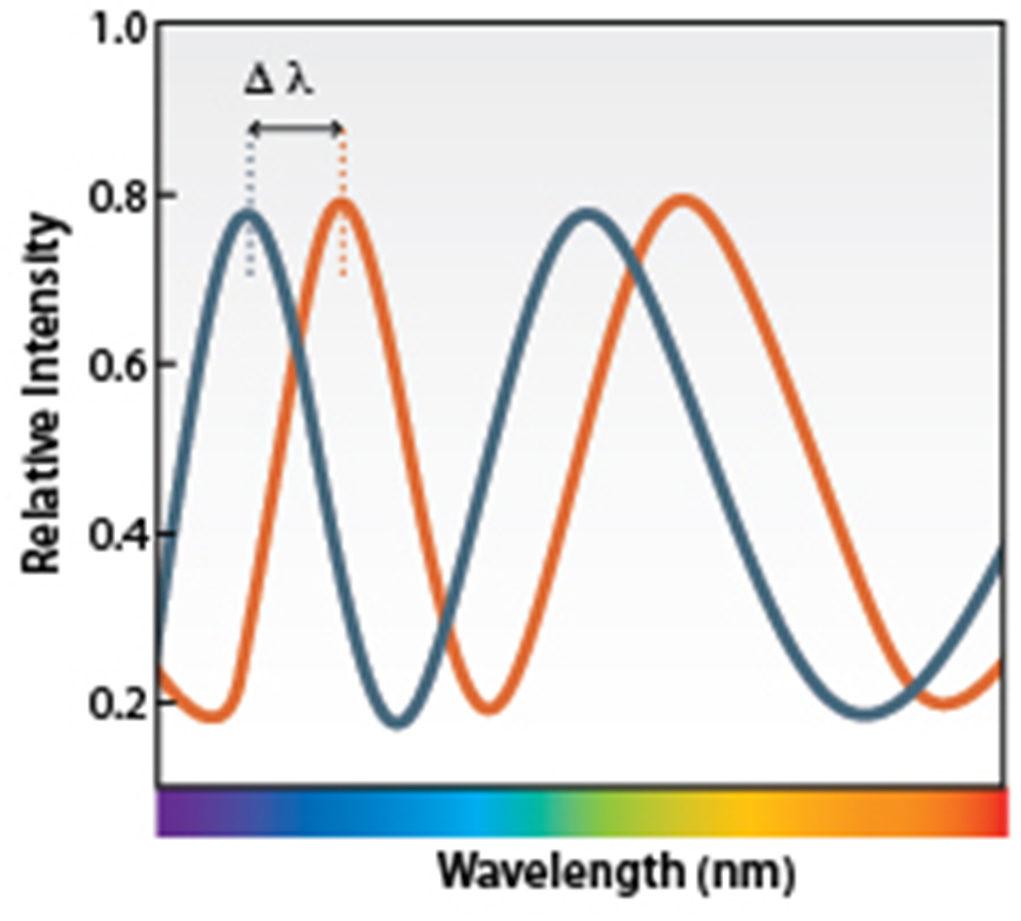 | Strengths: Monitors association and dissociation in real time of a soluble macromolecule to an immobilized species, with fidelity and accuracy. |
| Limitations: Requires immobilization of target protein with high stability. |
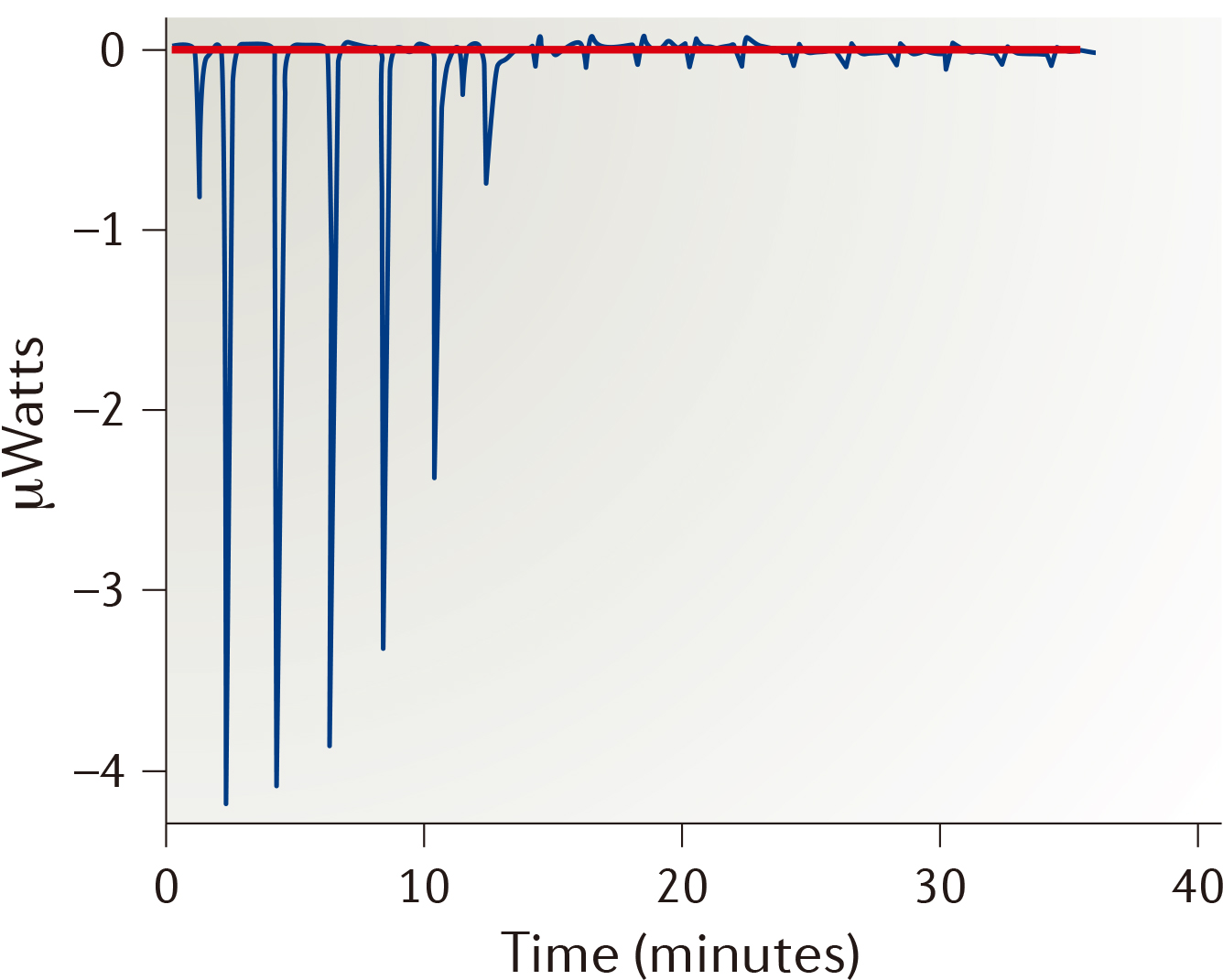 | Strengths: Directly determines the thermodynamic parameters for the binary system. |
| Limitations: Many titration components with high solubility are required and only suitable for binding events with an enthalpic component. |
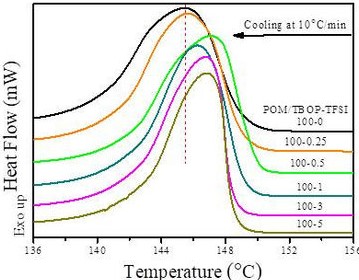 | Strengths: Determines the effects of a ligand on the thermal stability of a target protein. |
| Limitations: Requires large amounts of protein. |
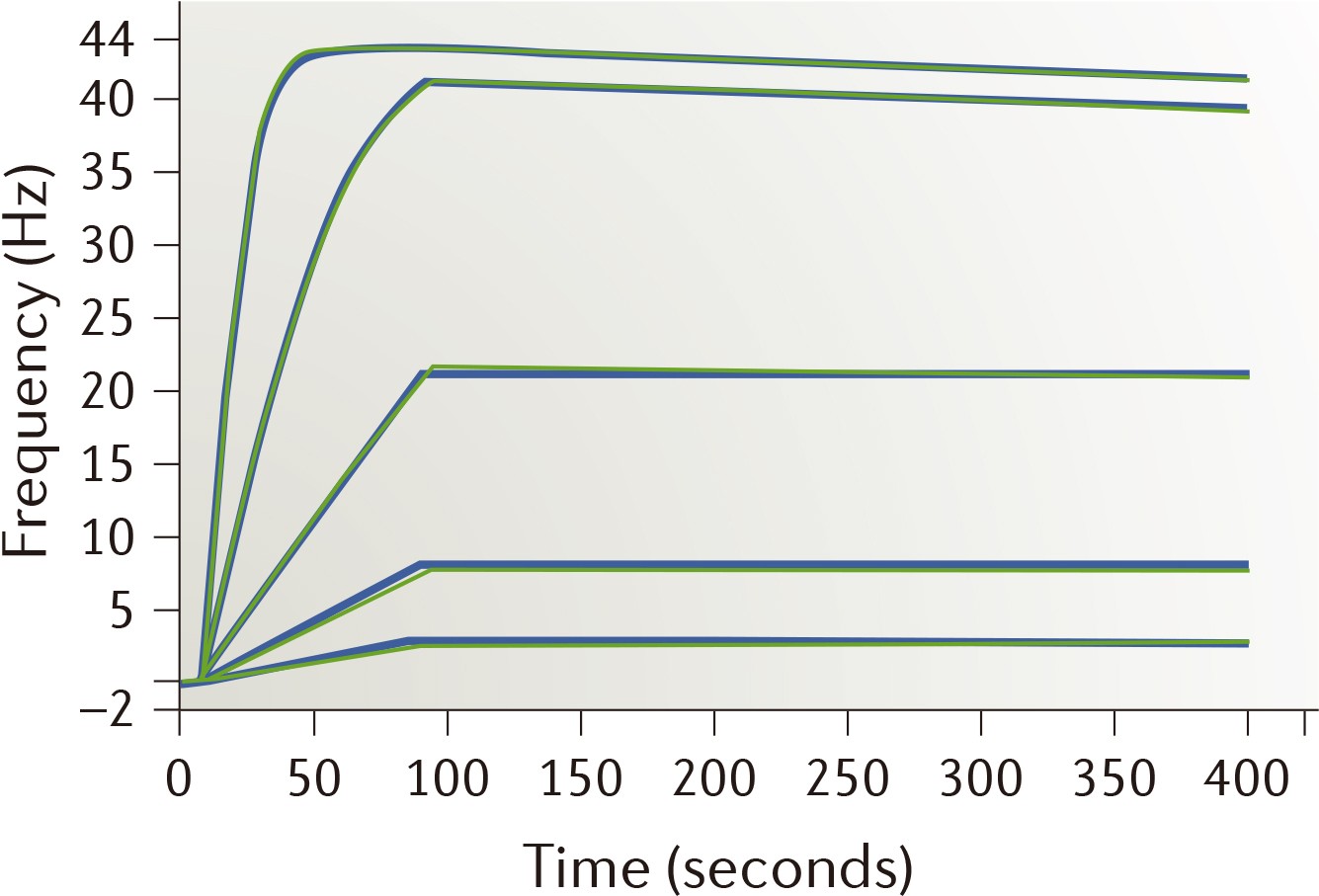 | Strengths: Time-resolved measurements of interactions with proteins and cells can be performed directly. |
| Limitations: Requires immobilization of target protein with high stability. |
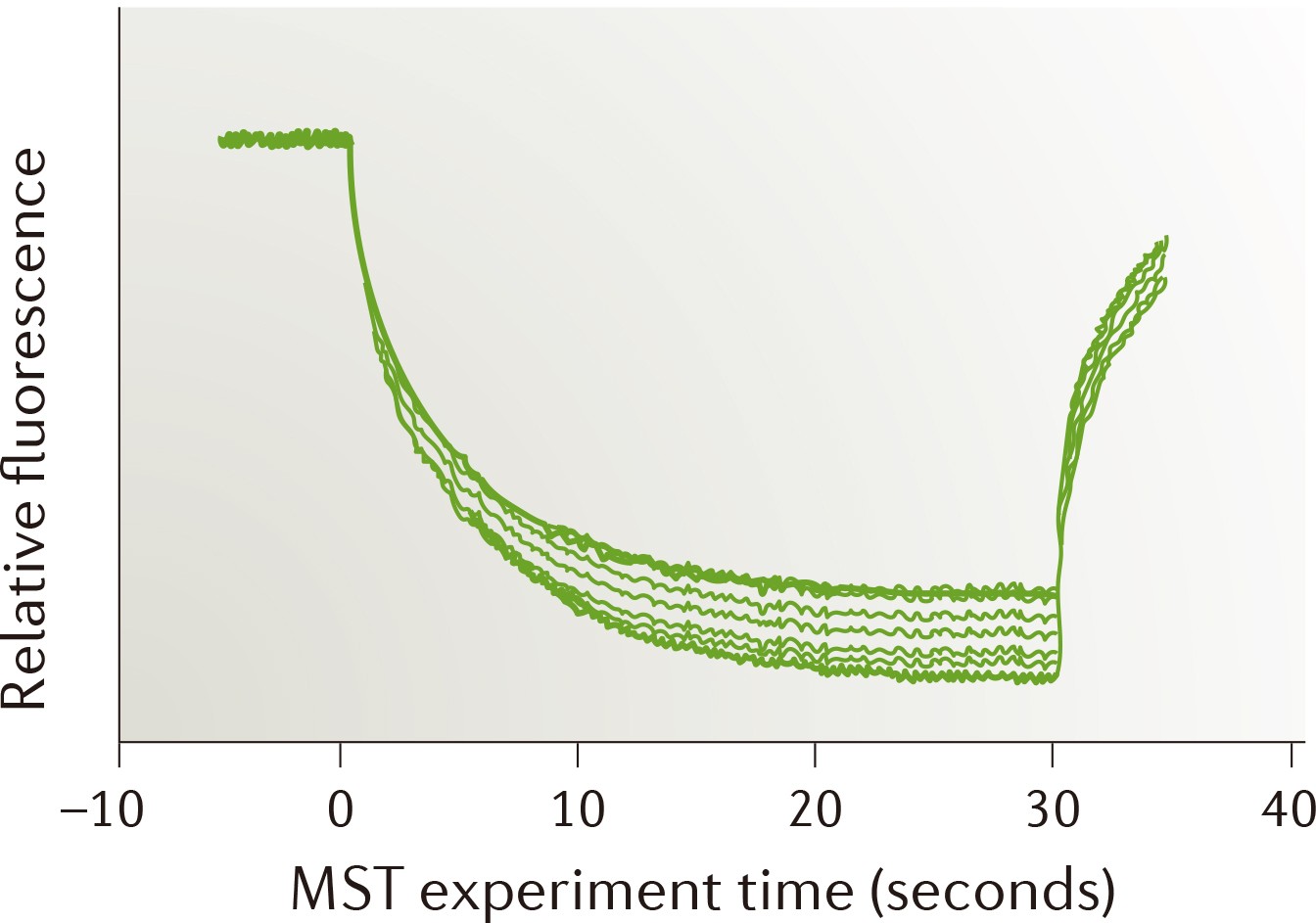 | Strengths: A non-immobilized approach for quantitative analysis of biomolecular interactions in solution. Suitable for measurements involving solubilized membrane proteins. |
| Limitations: Requires fluorescence labeling or strong intrinsic fluorescence. |
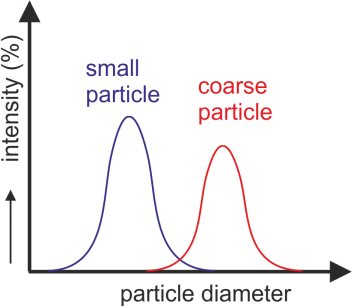 | Strengths: Characterizes the size of various particles, and to assesses the aggregation behavior of proteins, compounds, or protein-ligand binding complexes requiring little amounts of sample. |
| Limitations: Cannot distinguish closely related molecules owing to the low resolution and is restricted to the detection of transparent samples. |
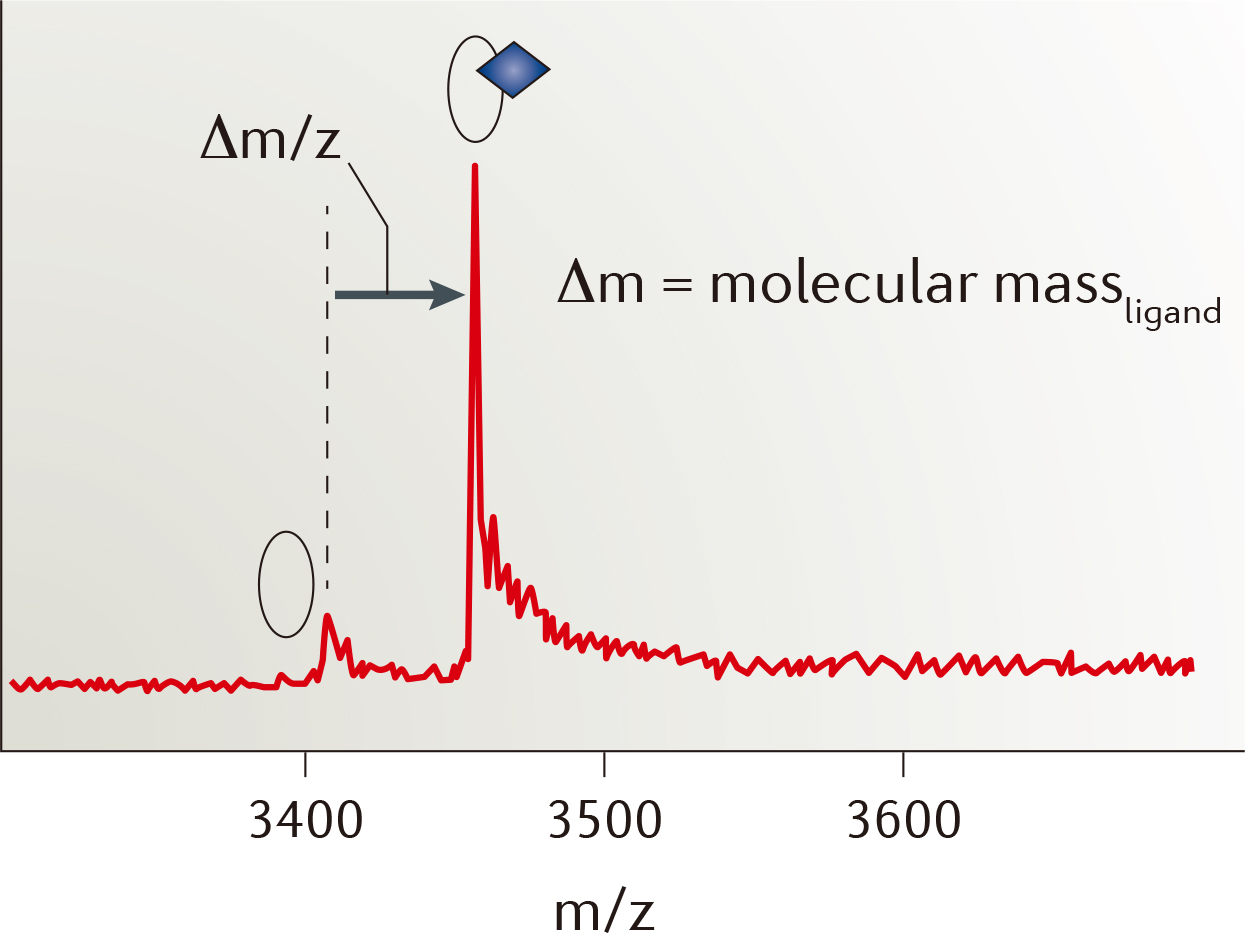 | Strengths: Directly visualizes complex formation and requires little amounts of label-free sample. We provide native MS, SEC-MS, and HDX-MS approaches for analyzing the protein-ligand interactions. |
| Limitations: The target protein must be highly stable in a buffer suitable for MS, and it is difficult to detect low-affinity binding. |
Capabilities and advantages of our Hit Biophysical Characterization services:
- We can use mainstream biophysical assays to yield information about binding specificity and stoichiometry as the basis for lead compound discovery and optimization.
- These approaches can be provided individually, and we can also offer a portfolio of multiple techniques to obtain comprehensive information.
- As a leading service vendor in the field of structural biology, we are confident of using structural biology approaches to provide structural information at the atomic level of the target-ligand complex to elucidate the binding mode.
- Our team of scientists can tailor cost-effective solutions for you according to specific project requirements and weigh the strengths and limitations of various biophysical techniques.
Creative Biostructure's experience in biophysical assays and expertise in interpreting data from hit biophysical characterization assist with lead discovery and optimization. We welcome scientists from academic institutions, biotechnology, and pharmaceutical industries to use our platforms to execute drug discovery related projects.
Reference
- Renaud J P.; et al. Biophysics in drug discovery: impact, challenges and opportunities. Nature Reviews Drug Discovery. 2016, 15(10): 679.
 Figure 1. Biophysical techniques in drug discovery. (Renaud J P.; et al. 2016)
Figure 1. Biophysical techniques in drug discovery. (Renaud J P.; et al. 2016)










