In the early stage of drug discovery, the kinetic and thermodynamic characterization of ligands that bind to specific target proteins can provide vital information for the identification and optimization of hit or lead compounds. The thermodynamics and kinetics of the protein-ligand association provide mechanistic insights into molecular interactions that determine the affinity of ligands to their specific target proteins. More detailed information besides the significant binding affinity can be obtained from thermodynamic characterization, such as the driving force for the ligand to associate with its target. The combination of protein-ligand structure information and thermodynamic parameters can help to fully understand the structure-activity relationship (SAR). Moreover, the kinetic parameters of protein-ligand recognition also reveal changes in binding events over time, which may be closely related to drug efficacy.
Creative Biostructure offers advanced isothermal titration calorimetry (ITC) services for researchers with drug discovery needs in academic institutions, biotech, and pharmaceutical industries around the world. The ITC is a powerful tool for measuring the thermodynamic parameters and binding kinetics of inter-molecular interactions without labeling, playing an important role in lead discovery and optimization processes.
A certain degree of thermal change occurs before and after the intramolecular and intermolecular biochemical reactions. The ITC technology exploits the principle of power compensation to accurately obtain the interactions of the system in a wide concentration range through single-concentration scanning. This approach directly measures the heat evolved or absorbed when the protein-ligand complexes are formed and complete the fitting calculation of combined thermodynamic parameters. The software can directly calculate the reaction binding enthalpy (ΔH), the constant pressure heat capacity (ΔCp), the binding equilibrium constant (Ka), the number of binding sites, as well as kinetic data between two or more molecules in the solution. Gibbs free energy (ΔG) and entropy change (ΔS) of the interaction can also be calculated.
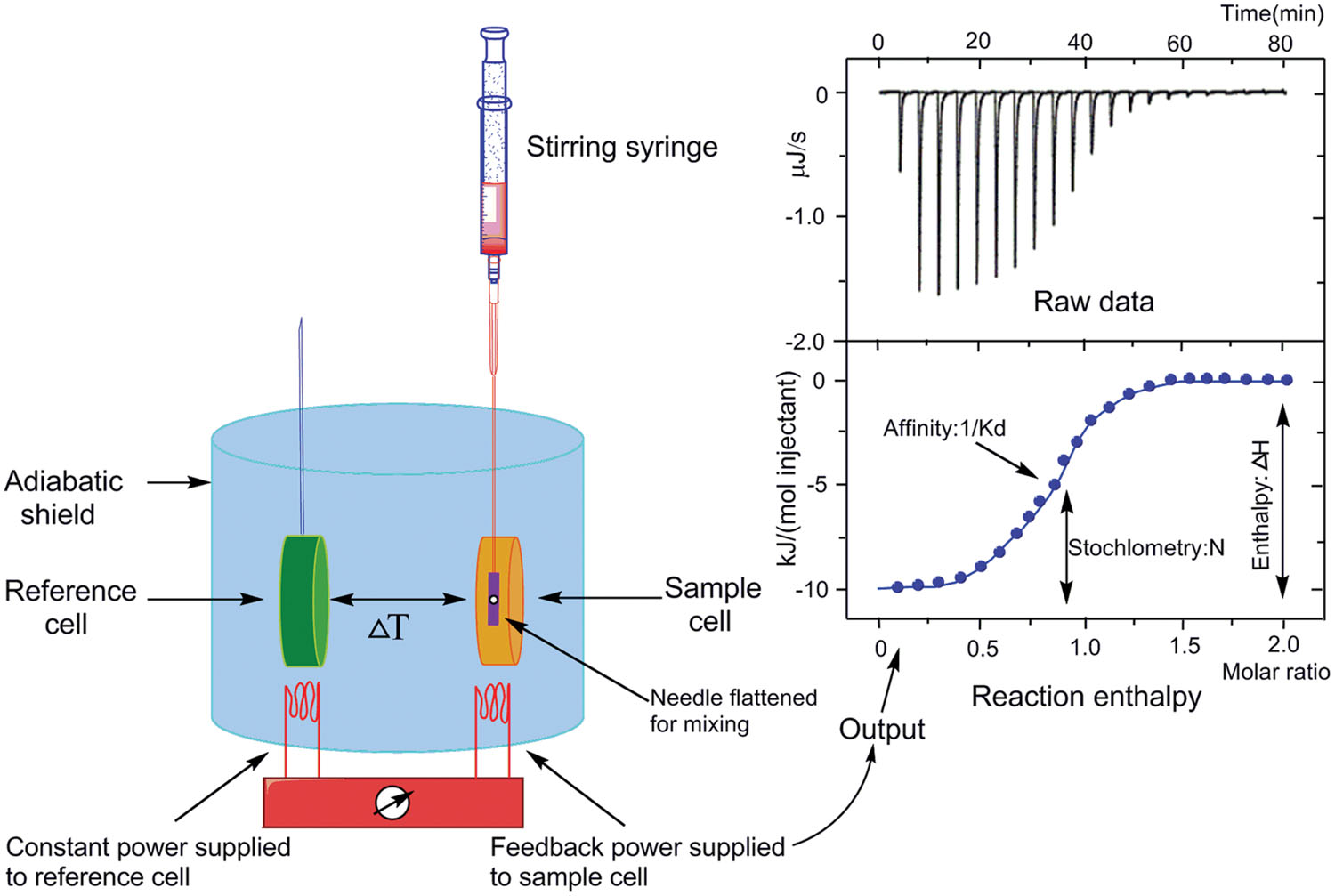 Figure 1. The schematic of ITC technology. (Song C.; et al. 2015)
Figure 1. The schematic of ITC technology. (Song C.; et al. 2015)
In short, ITC has multiple advantages in measuring the thermodynamic and kinetic parameters of ligands with proteins. For example, 1) It can simultaneously obtain the change of enthalpy (ΔH), entropy change (ΔS), the stoichiometry (n), and the binding constant (Ka) of the reaction through a single titration experiment; 2) The pan-assay interference compounds can be completely excluded, because ITC can directly measure the heat flow of protein-ligand binding without the fluorescence changes in many assays; 3) It enables to identify a promising hit/lead with a slow dissociation rate.
Creative Biostructure supports the entire process of ITC experimental design, assay development, verification, validation, and data analysis. The data obtained can not only serve as an important reference for the selection and optimization of hit/lead, but also provide molecular-level information for interpreting target-ligand binding events. Furthermore, we have established a variety of biophysical approaches based on advanced technologies for hit characterization. Want to know more? Please feel free to contact us for more information.
References

Easy access to products and services you need from our library via powerful searching tools