Optical biosensors have been utilized widely in many links and areas of drug discovery, such as target identification, ligand screening, lead compound selection, early ADME as well as manufacturing quality control. Among them, surface plasmon resonance (SPR) technology is a rapidly developing approach for determining binding events, which can characterize target protein-ligand interactions in real time. Creative Biostructure has extensive expertise and experience in SPR technology, and we can exploit this technology to provide sensitive, rapid, and reliable assays to help you measure real-time binding and kinetic information of ligands against specific targets. Our MagHelix™ SPR is a customizable solution that optimizes procedure for specific drug discovery projects, from experimental design to downstream applications.
SPR utilizes an optical approach to measure the refractive index change of the medium in close vicinity of a metal surface which can be applied to monitor the binding of ligands to receptors immobilized on the metal surface. This takes advantage of the phenomenon of surface plasmon generation in the thin metal films and the total internal reflection of light at the surface-solution interface, producing an electromagnetic film or evanescent wave which extends a short distance into the solution. Usually, the target protein is immobilized on the surface of the metal sensor, and then, the probe solution flows over the surface of the target, and if binding occurs, the probe will induce an increase in the refractive index. Therefore, SPR can determine the changes in reflected light before and after the probe binds to the target.
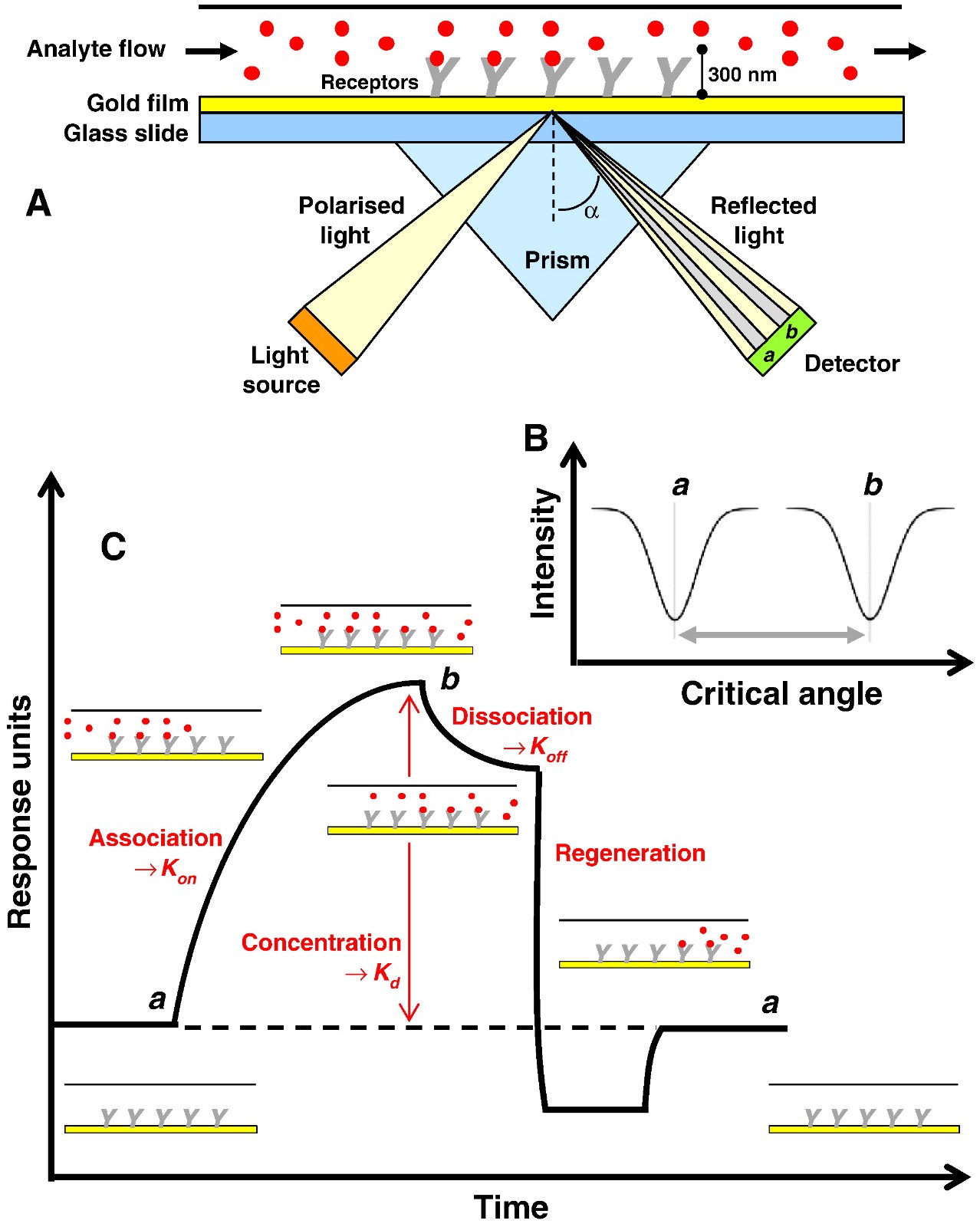 Figure 1. The schematic of SPR technology. (Patching S G. 2014)
Figure 1. The schematic of SPR technology. (Patching S G. 2014)
Briefly, the advantages of SPR include: 1) It is highly sensitive and suitable for detecting weak target-ligand interactions mediated by fragments; 2) Without labels and the detection of false positives caused by fluorescence quenching can be excluded; 3) Relatively small quantities of samples can be used for measuring real-time quantitative kinetics and binding affinity; 4) The medium-throughput mode makes it available as a tool for screening ligands. However, information about the binding site or pharmacophore of a ligand cannot be obtained.
At Creative Biostructure, we can offer corresponding compound libraries or fragment libraries for ligand screening, and we also provide other biophysical solutions to characterize the binding kinetics of hit compounds or fragments. Want to know more? Please do not hesitate to contact us for more information.
References

Easy access to products and services you need from our library via powerful searching tools