In addition to isothermal titration calorimetry (ITC), another microcalorimetry technique utilized to study protein-ligand interactions is differential scanning calorimetry (DSC). ITC measures the heat generated or consumed after titration of a ligand onto a target protein (or the reverse). The data obtained from an ITC experiment include Kd, stoichiometry, change in entropy (ΔS), change in enthalpy (ΔH), and the heat capacity change (ΔCp). DSC can measure the thermal changes and corresponding thermodynamics due to the thermal unfolding of a protein.
Creative Biostructure provides MagHelix™ Differential Scanning Calorimetry (DSC) services for studying the effect of ligands on the stability of target protein. In fact, as a powerful thermoanalytical technique, DSC is widely applied to study phase transitions, such as melting and exothermic decompositions, as well as glass transitions. It provides a great deal of information on the physical and energy properties of the studied substance. If you would like to accurately characterize drugs, monoclonal antibodies, nanostructured lipid carriers, or solid lipid nanoparticles, our DSC solutions can also meet your research needs.
DSC technology can study thermally induced transitions, especially conformational transitions in biomolecules, such as those between the folded and unfolded structure of a protein. This technique provides thermodynamic parameters associated with these conformational transitions, for example, ΔH°m, ΔCp, ΔG° (T), and ΔS° (T). It can also indirectly yield information about the stability of molecular associations. Compared with ITC, the approach of measuring binding constants using DSC technology is indirect, because the estimated binding constant is obtained by measuring the equilibrium between folded and unfolded proteins rather than that between bound and unbound forms. However, the technique is limited by the requirement for high concentrations of protein (≥ 1mg/mL). This may cause difficulties due to the aggregation of denatured proteins or the self-association of the native state. Therefore, accurate DSC studies need to assess the concentration dependence of thermodynamics.
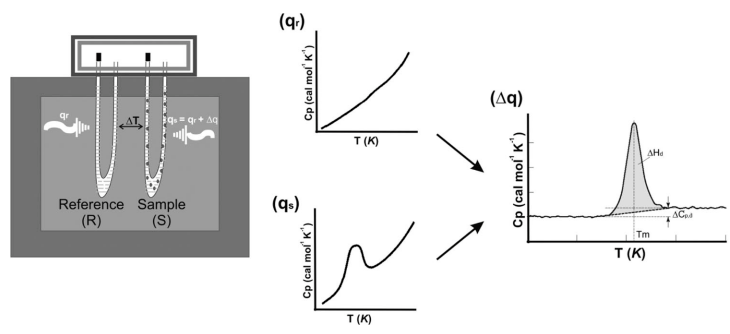 Figure 1. The schematic of differential scanning calorimetry (DSC). (Gill P.; et al. 2010)
Figure 1. The schematic of differential scanning calorimetry (DSC). (Gill P.; et al. 2010)
Since enthalpy change is a universal property of chemical processes, this technology can be applied to a variety of systems. DSC is widely used to assess the stability of biological macromolecules and protein folding, as well as to understand the interactions between biomolecules, such as the influence of the presence of ligands on protein stability.
Creative Biostructure provides many biophysical techniques involved in the drug discovery process, which can assist screen, confirm, and optimize weak binding compounds, and obtain kinetic and thermodynamic information in the binding events of ligands with specific targets. These techniques can be provided individually or applied in combination to compensate for the limitations of individual technology. If you would like more information, please contact us.
References

Easy access to products and services you need from our library via powerful searching tools