Rapid access to detailed structural information about drug targets and bound ligands is significant in the identification and optimization of lead compounds. The structural information of the protein-ligand complex can accelerate the optimization process of potential lead compounds and help to solve problems related to compound selectivity, pharmacokinetics, as well as patentability. X-ray crystallography has always been a major technology to provide high-resolution protein-ligand complex structures for the pharmaceutical industry. As a professional contract service provider in the field of structural biology, Creative Biostructure offers high-throughput crystallography techniques for lead compound identification and optimization to assist you to obtain a large number of target-ligand (such as fragments, compounds) complex structures in a short time. The crystal structure is useful for understanding the binding mode of ligands and thus help guide the optimization of the lead compound.
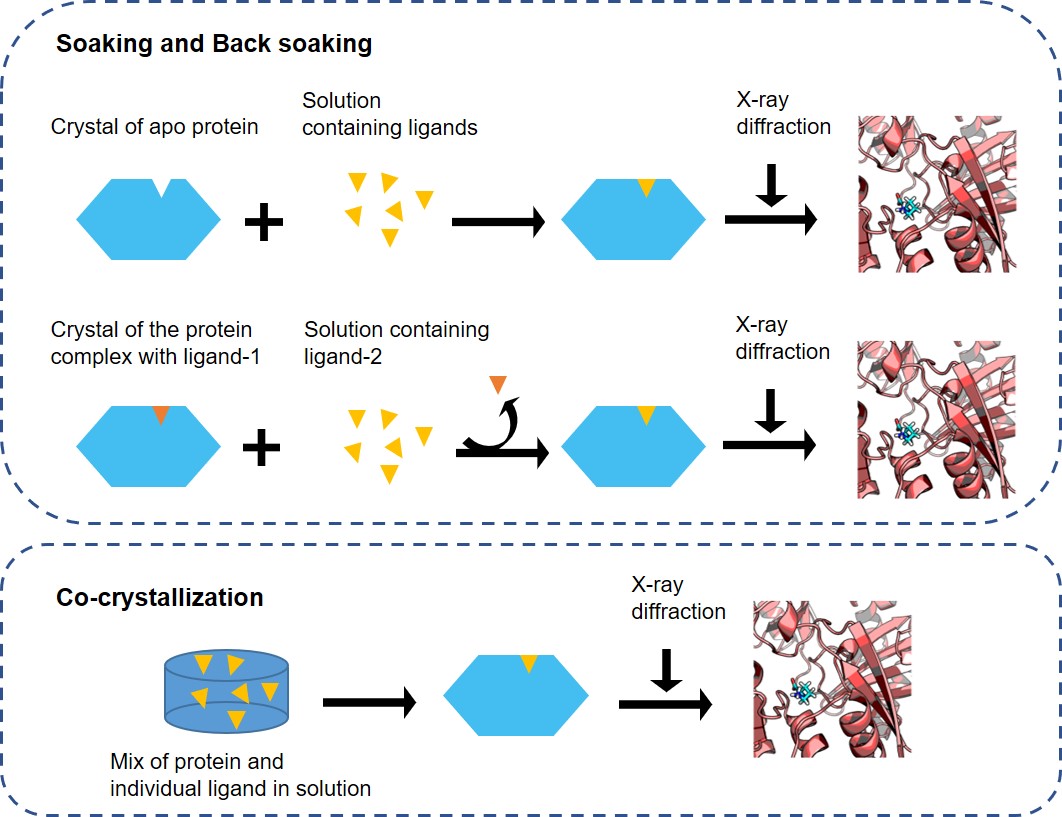 Figure 1. The schematic of soaking and co-crystallization.
Figure 1. The schematic of soaking and co-crystallization.
For any project that involves determining the structure of biological macromolecules, Creative Biostructure can provide flexible MagHelix™ Gene-to-Protein and Gene-to-Structure solutions. The steps of obtaining structures of protein-ligand complexes by X-ray crystallography include construct design and cloning, protein expression and purification, protein-ligand complex crystallization and crystal optimization, collection and processing of diffraction data, and refinement of the structure for several or dozens of target-ligand complexes. Among them, our crystallographic approaches for rapidly producing structural information of complexes include but are not limited to:
Soaking is the preferred approach for obtaining complex crystals if there is a soakable crystal form and the ligand has the appropriate solubility and intrinsic potency in soaking buffer. We will soak the ligand in the ligand-free (apo) form of the target protein crystal or replace the ligand previously bound to the target with the selected ligand. The soaking approach provides minimal obstacles to ligand binding.
Compared with soaking, protein-ligand co-crystallization usually requires more resources and time. However, it is often the best option when obvious conformational changes or crystal packing preclude other approaches. The requirement to optimize crystals for each complex makes this method resource- and time-intensive. Sometimes, the size or the chemical properties of the ligand may even require screening for new crystallization conditions.
Capabilities and advantages of our MagHelix™ Co-crystallization and Soaking services:
In addition to X-ray crystallography, Creative Biostructure can also provide a variety of other mainstream and novel biophysical techniques for the validation and characterization of hit compounds. We welcome scientists from academic institutions and biotech/pharmaceutical industries with drug discovery requirements to exploit our platform to execute related projects.
References

Easy access to products and services you need from our library via powerful searching tools