Once the most promising hit compounds have been identified as leads through the efforts of hit to lead, it will enter the stage of lead optimization and preclinical development of early drug development, which are the key processes for finalizing drug candidates entering clinical trials. Lead optimization aims to improve the properties of lead compounds including stability, potency, efficacy, exposure, safety, etc. Lead optimization involves a sophisticated iterative stage in which the bioactivity and drug activity of the lead series are extensively optimized through various screening filters, and development candidates are ultimately determined for preclinical development. Preclinical development, also known as IND-enabling studies, involves testing development candidates in various in vitro and in vivo assays to examine their safety pharmacology and toxicology profile.
With established in vitro/in vivo approaches and our advanced zebrafish screening platform, Creative Biostructure can start from the identified potential lead compounds to study stability, bioavailability, toxicity, efficacy, and other pharmacokinetic parameters to improve their target specificity and potency. Our lead optimization and preclinical development solutions can provide you with better quality leads that have a high probability of success in clinical development.
 Drug development is a high-risk investment, especially when entering the clinical development stage, developers are faced with high costs and increasing attrition rates. Therefore, early determination of the absorption, distribution, metabolism, excretion (ADME), and toxicological (Tox) properties of the lead series is an effective strategy to balance the risk-reward ratio and improve project productivity. Creative Biostructure agrees with this view of “fail early and fail cheap”, and we provide in vitro ADME-Tox profiling solutions for global customers with drug development needs to evaluate the ADME-Tox properties of drug candidates and prioritize them. We have a well-trained and knowledgeable drug discovery team, and we have gained extensive in vitro ADME-Tox expertise and experience through cooperation with academic institutions, biotech, and pharmaceutical companies.
Drug development is a high-risk investment, especially when entering the clinical development stage, developers are faced with high costs and increasing attrition rates. Therefore, early determination of the absorption, distribution, metabolism, excretion (ADME), and toxicological (Tox) properties of the lead series is an effective strategy to balance the risk-reward ratio and improve project productivity. Creative Biostructure agrees with this view of “fail early and fail cheap”, and we provide in vitro ADME-Tox profiling solutions for global customers with drug development needs to evaluate the ADME-Tox properties of drug candidates and prioritize them. We have a well-trained and knowledgeable drug discovery team, and we have gained extensive in vitro ADME-Tox expertise and experience through cooperation with academic institutions, biotech, and pharmaceutical companies.
We can provide a wide range of in vitro ADME-Tox services, including physicochemical profiling, in vitro ADME and PK, and toxicological assessments. We offer flexible approaches for ADME-Tox profiling, with many tests available in multiple formats, allowing you to select different throughput and service portfolio based on project requirements. Advanced equipment allows high-throughput in vitro screening, which is time-saving and cost-effective. Moreover, we can combine the experimental results to optimize the lead structure.
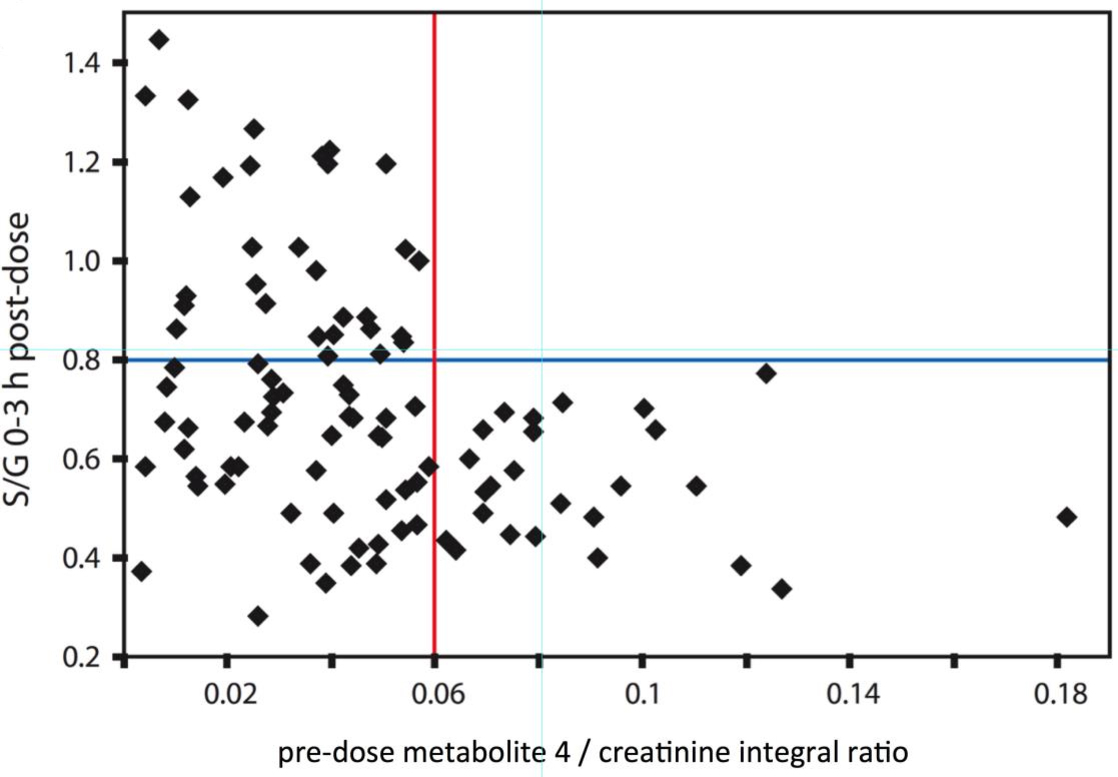 In the process of drug development, the use of animal models to evaluate the efficacy of drug candidates is an indispensable link. To ensure the data quality of preclinical studies, researchers need to select the appropriate preclinical animal model. Creative Biostructure can exploit NMR-based pharmacometabonomics technology to screen the most suitable animal model for you through the NMR spectrum. For example, in the study of dyslipidemia, we utilized this method to investigate and find that the metabolic syndrome of the rhesus monkeys is most similar to that of humans.
In the process of drug development, the use of animal models to evaluate the efficacy of drug candidates is an indispensable link. To ensure the data quality of preclinical studies, researchers need to select the appropriate preclinical animal model. Creative Biostructure can exploit NMR-based pharmacometabonomics technology to screen the most suitable animal model for you through the NMR spectrum. For example, in the study of dyslipidemia, we utilized this method to investigate and find that the metabolic syndrome of the rhesus monkeys is most similar to that of humans.
The application of metabonomics technology in pharmaceutical research has developed pharmacometabonomics and has been successfully applied in various aspects and stages of pharmaceutical research, such as individualized drug therapy, drug toxicity evaluation, and safety warning. In addition, animal model screening has become an important aspect of the preclinical study of pharmacometabonomics. Our experts in NMR spectroscopy and pharmacometabonomics can select appropriate animal pharmacology and disease models according to your project requirements, and also help you analyze the targets and processes of drug action from the perspective of metabolic network regulation and reveal the mechanism of action of drug candidates.
 The zebrafish model system not only has the characteristics of in vitro cell-based assays, such as low drug consumption, low cost, and high throughput, but also has the characteristics of animal experiments, allowing observe multiple organs, evaluate pharmacodynamics and pharmacokinetics, and assess metabolite activity and so on. Therefore, zebrafish is an ideal vertebrate model for rapid, cost-effective, and highly predictive evaluation, and can be utilized as a platform for small molecule screening, drug efficacy, and toxicity testing. With extensive experience and a leading technology platform, the experts of Creative Biostructure can provide you with the modeling and validation of zebrafish disease models for drug development. Our zebrafish models have been successfully used to evaluate the developmental and teratogenic toxicity, cardiovascular toxicity, hepatotoxicity, and renal toxicity of drugs.
The zebrafish model system not only has the characteristics of in vitro cell-based assays, such as low drug consumption, low cost, and high throughput, but also has the characteristics of animal experiments, allowing observe multiple organs, evaluate pharmacodynamics and pharmacokinetics, and assess metabolite activity and so on. Therefore, zebrafish is an ideal vertebrate model for rapid, cost-effective, and highly predictive evaluation, and can be utilized as a platform for small molecule screening, drug efficacy, and toxicity testing. With extensive experience and a leading technology platform, the experts of Creative Biostructure can provide you with the modeling and validation of zebrafish disease models for drug development. Our zebrafish models have been successfully used to evaluate the developmental and teratogenic toxicity, cardiovascular toxicity, hepatotoxicity, and renal toxicity of drugs.
 Zebrafish is an important vertebrate model for studying the safety and/or efficacy of targeted compounds on different stages of development. Identifying potential toxicity in drug candidates early in the drug development process contributes to reducing the overall cost of the drug discovery phase. Creative Biostructure utilizes the zebrafish model to detect the toxicity of drug candidates. Our toxicity assays include but are not limited to acute toxicity assay, reproductive/developmental toxicity assay, cardiotoxicity assay, hepatotoxicity assay, neurotoxicity assay, ototoxicity assay, and multi-organ toxicity assay. We not only offer zebrafish model-based toxicity assays for researchers with drug toxicity assessment needs but also provide in silico toxicity prediction and high-throughput in vitro toxicity profiling.
Zebrafish is an important vertebrate model for studying the safety and/or efficacy of targeted compounds on different stages of development. Identifying potential toxicity in drug candidates early in the drug development process contributes to reducing the overall cost of the drug discovery phase. Creative Biostructure utilizes the zebrafish model to detect the toxicity of drug candidates. Our toxicity assays include but are not limited to acute toxicity assay, reproductive/developmental toxicity assay, cardiotoxicity assay, hepatotoxicity assay, neurotoxicity assay, ototoxicity assay, and multi-organ toxicity assay. We not only offer zebrafish model-based toxicity assays for researchers with drug toxicity assessment needs but also provide in silico toxicity prediction and high-throughput in vitro toxicity profiling.
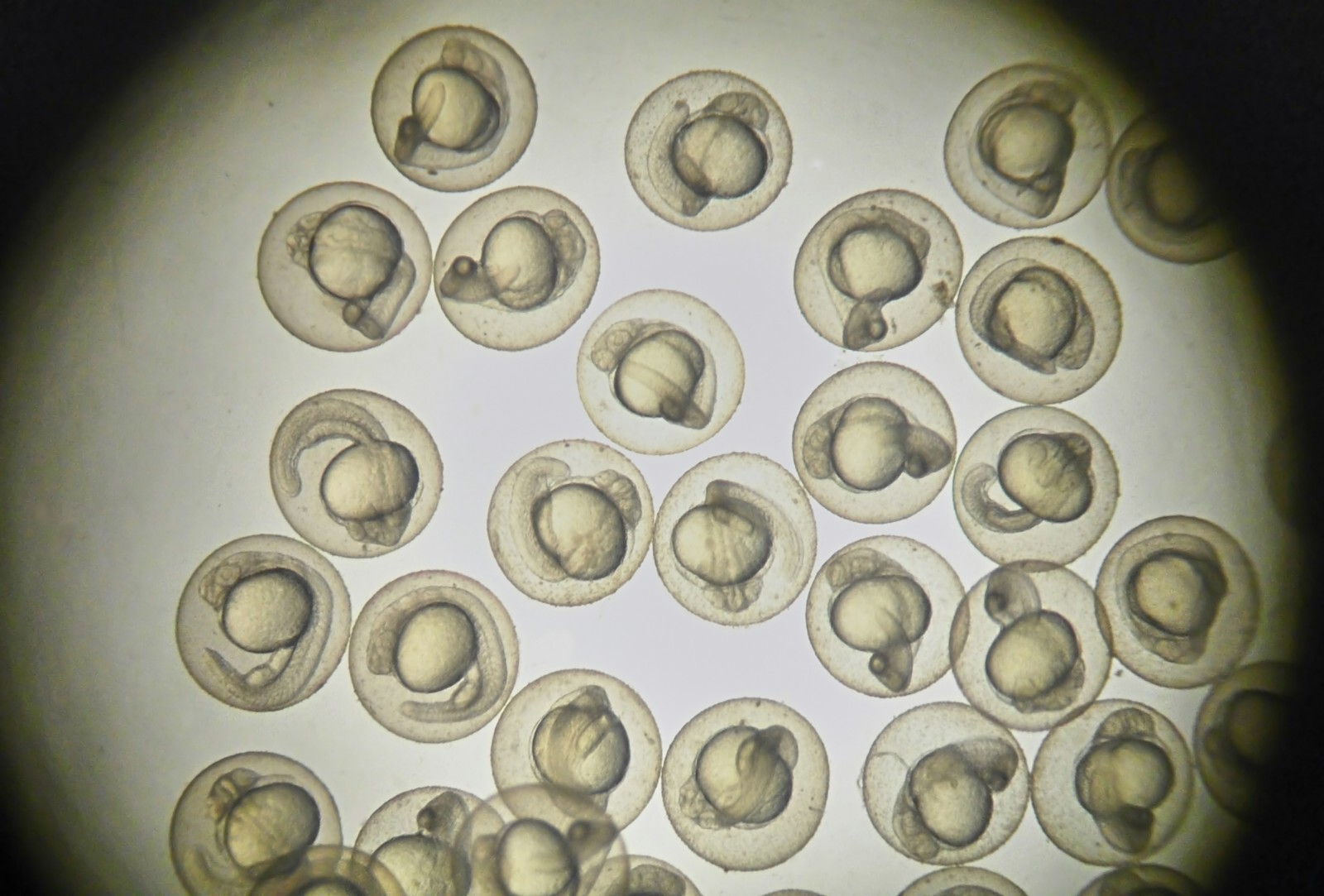 In vivo zebrafish studies can bridge the gap between in vitro cellular assays and in vivo disease models of higher mammal. Therefore, zebrafish has become one of the best living models for human disease research and in vivo high-throughput drug screening. The zebrafish model system can predict the efficacy of drug candidates, thereby further ensuring that only the most optimized lead compounds are developed in the drug discovery value chain. Creative Biostructure can provide high-throughput in vivo drug screening and drug efficacy evaluation for a variety of diseases. Our platform covers various drug efficacy evaluation models, such as the evaluation model of agents for inhibiting angiogenesis and cardioprotection models for evaluating the cardioprotective potential of drug candidates. In addition, we support the development of new zebrafish models to assess the adverse reactions and efficacy of drug candidates.
In vivo zebrafish studies can bridge the gap between in vitro cellular assays and in vivo disease models of higher mammal. Therefore, zebrafish has become one of the best living models for human disease research and in vivo high-throughput drug screening. The zebrafish model system can predict the efficacy of drug candidates, thereby further ensuring that only the most optimized lead compounds are developed in the drug discovery value chain. Creative Biostructure can provide high-throughput in vivo drug screening and drug efficacy evaluation for a variety of diseases. Our platform covers various drug efficacy evaluation models, such as the evaluation model of agents for inhibiting angiogenesis and cardioprotection models for evaluating the cardioprotective potential of drug candidates. In addition, we support the development of new zebrafish models to assess the adverse reactions and efficacy of drug candidates.
Reference

Easy access to products and services you need from our library via powerful searching tools