In drug discovery, biophysical approaches have become attractive screening techniques. They are not only the main primary hit finding methodologies, such as weakly active fragments, but also as orthogonal approaches for the validation of hit compounds found by conventional biochemical or cellular assays. Among them, thermal shift assay (TSA) is a reliable and simple technique to identify active fragments and analyze the binding events of ligands to the target by monitoring changes in protein thermal stability.
Creative Biostructure can provide appropriate TSA solutions according to the specific requirements of drug discovery projects, including but not limited to reporter dyes detection methods (thermofluor, thiol-specific reaction dyes CPM, rigidity sensitive dyes DCVJ), measurement of the intrinsic tryptophan fluorescence lifetime, (high-throughput) cellular thermal shift assay (CETSA), fast parallel proteolysis (FastPP), radioligand binding thermostability assay (RBTA), as well as fluorescence-detection size-exclusion chromatography-based thermostability assay (FSEC-TS).
The stability of a protein is related to its melting temperature, which is affected by conditions such as buffer composition, pH values, amino acid mutations, or ligand/fragment binding. Fluorescence-based thermal shift assay (usually referred to as differential scanning fluorimetry or the thermofluor assay) is one of the common TSA techniques and is a high-throughput and economical biophysical approach for screening and validation of hits. In general, the fluorescence of the protein solution is measured under a temperature gradient. As the temperature rises, the hydrophobic core of the protein is exposed, and the added fluorescent dye has affinity for the hydrophobic part of the protein. Therefore, this leads to an increase in the fluorescence signal and can be used to determine the melting temperature of the protein. The interaction between ligands and proteins can significantly increase or decrease the melting temperature. Thus, a change of this parameter offers crucial information about protein stabilization or destabilization.
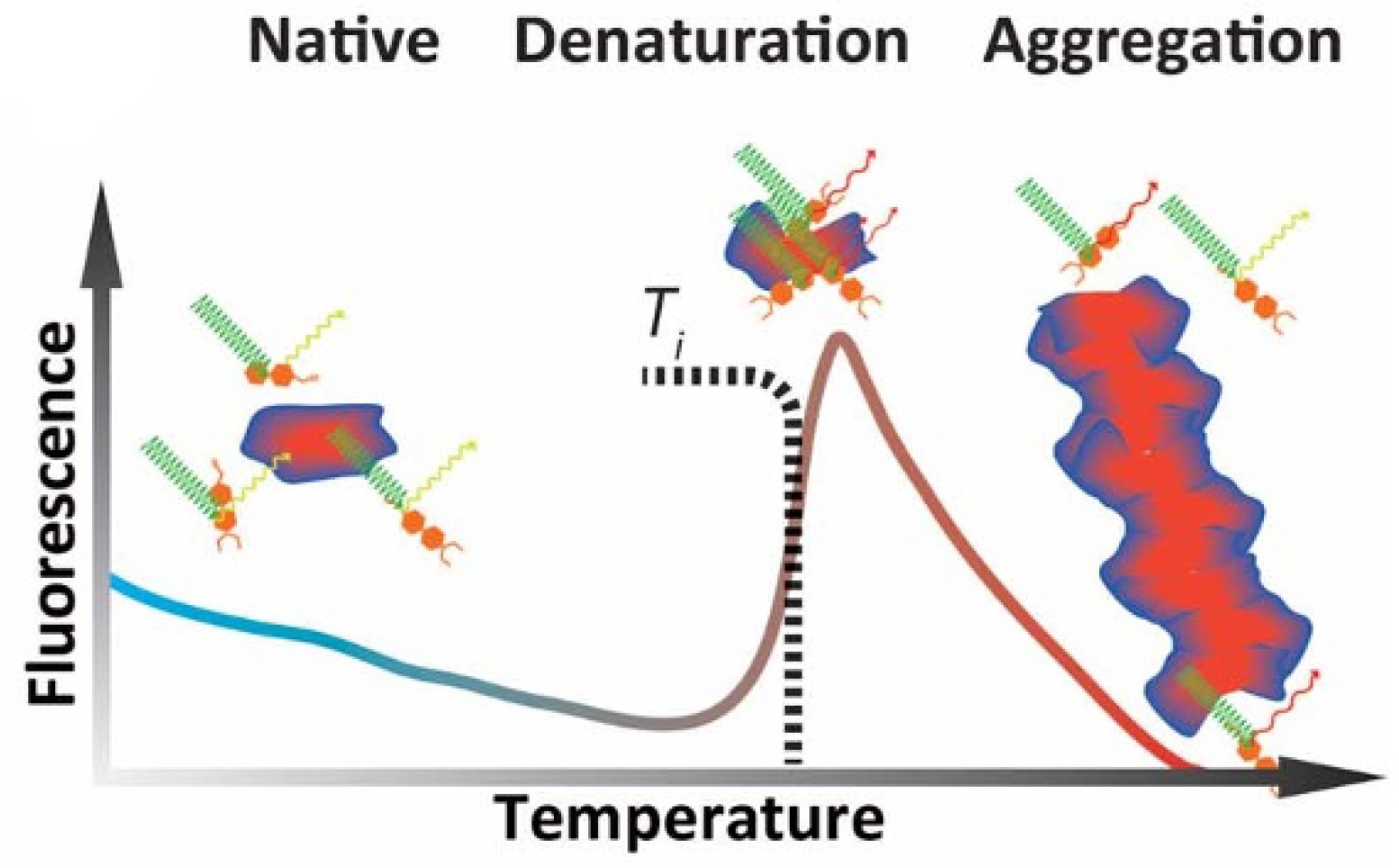 Figure 1. Different states of a protein and its exogenous fluorescence response. (Vollrath F.; et al. 2014)
Figure 1. Different states of a protein and its exogenous fluorescence response. (Vollrath F.; et al. 2014)
In addition to Thermofluor, other TSA approaches that analyze protein-ligand binding events by monitoring changes in protein thermal stability, such as approaches based on non-fluorescent dyes CPM, the intrinsic tryptophan fluorescence lifetime/wavelength, static light scattering, FastPP, CETSA, FSEC-TS, RBTA, etc., all of which have strengths and limitations.
In addition to TSA approaches, Creative Biostructure can also provide other biophysical techniques for the identification, validation, affinity, selectivity, and binding mechanisms of hits involved in early drug discovery. We support combinatorial approaches, such as combining TSA assays with surface plasmon resonance (SPR) for independent verification of biophysical parameters. Please feel free to contact us if you would like to learn more.
References

Easy access to products and services you need from our library via powerful searching tools